DVA - Concepts In Molecular Medicine, April 8 - 13, 1996
Course Outline
by
Josiah N. Wilcox, Ph.D.
Department of Medicine, Division of Hematology/Oncology,
Emory University, Atlanta, GA 30322
B. BASIC IN SITU HYBRIDIZATION PROTOCOL (continued)
5. Hybridization
6. Modifications for Paraffin Sections or Cultured Cells
7. Hybridizations Using Synthetic Oligomers
8. Controls
9. Non-radioactive In Situ Hybridization
10. Size Reduction of Riboprobes
11. In Situ PCR
12. In Situ PCR or PCR and In Situ Hybridization?
Hybridization:
Once the probe and slides have been prepared it is relatively easy to hybridize as many as 100-200 slides per day. The frozen sections are removed from the freezer and collected on a metal slide tray. The slides are then thawed and dried by incubating them for 5min exactly at 50°C. The drying at this step has been found to improve tissue retention on the slide throughout the hybridization procedure. After drying the slides are put into plastic slide carriers (each holding 25 slides) and immersed in 4% paraformaldehyde for 10min at 4°C to ensure that the tissue is equally fixed. This fixation also tends to improve tissue retention on the slide as well. After fixation the slides are washed in 0.5xSSC (1xSSC= 150mM NaCl 15mM sodium citrate, pH 7.0) and permeabilized in 1.0ug/ml proteinase K (Sigma) in 500mM NaCl 10mM Tris, pH8.0 for 10min at room temperature. This is important as permeabilization with proteinase K, pronase, or HCl have been shown to improve the signal intensity after in situ hybridization presumably because they either loosen up the membranes allowing greater penetration of the probes or because they partly digest proteins associated with the RNA allowing longer regions access to the probes (Armstrong et al 1992; Wilcox et al 1986).
The amount of proteinase K used needs to be optimized for each lot of proteinase K. It is suggested that this is tested by doing a mock hybridization with a range of proteinase K concentrations prior to starting actual experiments. A good starting point is that concentration of proteinase K which provides the best final tissue morphology and tissue retention on the slides at the end of the mock hybridization procedure. Once a hybridization signal is obtained it would be appropriate to go back and then optimize the signal by again testing various concentrations of proteinase K. One of the biggest problems with the technique as mentioned earlier is keeping the sections on the slide throughout the hybridization procedure. If this becomes a serious problem the first thing to do is eliminate the proteinase K step. It is important to remember that while proteinase K improves the signal intensity it is not necessary to get a signal in the first place.
After the proteinase K treatment the slides are washed for 10min in 0.5xSSC, dried carefully around the section with a lint-free tissue, and placed in air-tight hybridization boxes (Nalgene utility boxes, Baxter Health Care Products #L1995-4) containing filter paper saturated with box buffer (4xSSC, 50% formamide). The prehybridization is begun by pipetting a small amount of hybridization buffer (100µl) onto the sections (for riboprobes rHB2: 10mM DTT, 0.3M NaCl, 20mM TRIS, pH8.0, 5mM EDTA, 1x Denhardt's, 10% Dextran Sulfate, 50% Formamide; for oligomers HB8: 10mM DTT, 1x Denhardt's, 5xSSC, 100µg/ml ssDNA, 100µg/ml tRNA, 10% Dextran Sulfate, 20% Formamide). The tissues are prehybridized for 1-3hrs at 42°C and then the hybridization begun by adding 20µl of a probe mixture which is pipetted carefully into the bubble of prehybridization solution covering the tissue section. The hybridization mixture contains 2µl 35S-labeled riboprobe (300,000 cpm/µl in 1xTE), 1µl tRNA (50mg/ml stock in dH2O), and 17µl rHB2 for each 100µl of prehybridization buffer on the slides. This is prepared by adding the probe and tRNA to a microfuge tube, heating this at 95°C for 3 min in a heat block, followed by the immediate addition of ice cold rHB2. The solution is then vortexed and kept on ice until used for the hybridization. Once the probe mix is added to the slides the boxes are covered again and placed in a 55°C incubator overnight. Coverslipping of the slides is not necessary nor is it recommended during the hybridization incubation. The surface tension of the hybridization buffer is sufficient to keep the probe mixture in contact with the tissue overnight and as long as the hybridization is carried out in sealed boxes with filter paper saturated with 4xSSC 50% formamide the tissue sections will not dry out. Coverslipping has its own problems with air bubbles and potential damage to the tissue or radioactive contamination of the lab upon removal of the coverslips after the hybridization is complete.
Extreme care should be taken to ensure that all buffers, reagents, slide holders are ribonuclease (RNase) free up to and including the hybridization at step 8. RNase contamination is not a great problem in the post-hybridization steps when using riboprobes since the tissues will be treated with RNase as part of the stringency wash. It is for this reason that care should be taken that all of the slide holders and beakers used after step 8 are kept for that purpose alone and never used during the prehybridization procedure since these materials will probably remain contaminated with RNase. It is suggested that all equipment and containers used for the prehybridization steps be periodically treated with diethyl pyrocarbonate (DEPC, Sigma). This is done by soaking the equipment in fresh 0.1% solution of DEPC overnight in the fume hood. The DEPC should be diluted in sterile dH2O and used immediately as it degrades quickly.
The hybridization reaction is essentially complete after 4 hrs but it is convenient to let the incubation go overnight prior to beginning the post-hybridization washes. The slides are removed from the hybridization boxes and rinsed twice for 10 min each in 2xSSC containing 10mM beta-mercaptoethanol and 1mM EDTA (ßME/EDTA) at room temperature to wash off the majority of radioactive probe associated with the slides. This is followed by RNase A treatment (Sigma; 20µg/ml in 500mM NaCl, 10mM Tris, pH8.0) for 30min at room temperature. RNase A will attack single stranded RNA but spare RNA-RNA duplexes and so ensures that the probe is properly complexed to the mRNA by complementary base pairing. RNase treatment will significantly reduce the specific signal but will help provide the extremely low backgrounds that enable the detection of very low copy number mRNAs in tissue using this technique.
Great care needs to be taken with the handling of the RNase at this stage to prevent any cross contamination of pipettes or equipment which will cause problems with later hybridizations. It is for this reason that all equipment used in the post-hybridization washing steps is kept completely separate from material used for the pre-hybridization steps. The RNase treatment is followed by two more 2xSSC ßME/EDTA washes at room temperature and then a high stringency wash in 4 liters of 0.1xSSC ßME/EDTA at 55°C for 2 hrs. This can be done in a large 4 liter beaker on a stir plate which is heated with an immersion heater. This is followed by 2 washes with 0.5xSSC without ßME/EDTA at room temperature. The ßME/EDTA is added to keep the 35S-labeled probe in a reduced state throughout the washing steps which may reduce backgrounds. It is important however to remove the ßME/EDTA before coating the sections with photographic emulsion since reducing agents would cause chemical reduction of the silver salts and high background.
Finally the tissue sections are dehydrated in graded alcohols containing 0.3M NH4Ac, dried in a vacuum desiccator and can be stored desiccated at room temperature until coating with photographic emulsion. The slides are exposed for varying lengths of time in the dark in sealed slide boxes with desiccant at 4°C. The exposure time may vary depending on the amount of mRNA in the tissue. This can range from 3 days for an abundant mRNA (proopiomelanocortin in the rat pituitary) (Wilcox et al 1986) to 8 wks for platelet-derived growth factor expression in human vascular tissue (Wilcox et al 1988) or even 12 wks for rare mRNAs like tumor necrosis factor or tissue plasminogen activator (Gordon et al 1989). Since it is not always possible to know the optimum exposure time for each tissue or probe it is a good idea to perform all hybridizations in triplicate and develop one slide every 4 wks until the optimum exposure is determined.
Modifications for paraffin sections, or cultured cells:
The same basic protocol described in the preceding sections can also be used for paraffin sections or cultured cells with slight modifications. Paraffin embedding after paraformaldehyde fixation provides good preservation of the cellular mRNA for hybridization. However, direct comparison of in situ hybridization expreriments using paraffin or frozen blocks from the same tissue indicates that there is approximately a 25% loss in hybridization signal due to paraffin (Smith and Wilcox, unpublished observations). Consequently, where paraffin embedding is necessary for appropriate tissue morphology (i.e. lung), use it and plan on a slightly longer exposure time to make up for the weaker signal. Prior to hybridization the paraffin is removed by washing twice for 2min in xylene, twice in 100% ethanol for 1min, for 1min each in 95% ethanol, 70% ethanol, 50% ethanol and 0.5xSSC. The sections are then ready for hybridization in the frozen section protocol beginning with the paraformaldehyde fixation at step 2. In the same manner cultured cells can be fixed and prepared for hybridization. Cells are either grown on Lab-Tek (Baxter) culture slides or centrifuged onto Vectabond coated or Superfrost Plus slides and then fixed by immersion in 4% paraformaldehyde at 4°C for 10min. The slides are then washed briefly (30sec) and stored in 70% ethanol at 4°C for up to 3 months. Prior to hybridization the slides are taken from the ethanol and then enter the hybridization procedure starting with step 3 in the frozen section protocol by washing the slides in 0.5xSSC.
Hybridizations using synthetic oligomers:
We and others have used synthetic oligomers for in situ hybridization studies with great success. The advantage of oligomers as probes comes primarily from their availability (ie. "Clone-by-Phone"). Oligomers specific for many mRNAs can be obtained commercially from a number of companies (Boehringer Mannheim, Oncogene Science, others) or custom synthesized based on the sequence information available from GeneBank or published articles, also by commercial laboratories, for as little as $1.00 a base. The oligomers we use are typically 40-50 bases long and can be used in the same protocol as for 35S-labeled riboprobes on frozen sections with the following modification: HB8 (see buffers) is used in place of rHB2 as the hybridization buffer; the oligos are labeled by a tailing reaction using 35S-labeled CTP (see methods) and the hybridization mix (step 7a) consists of 1µl probe (start with 300,000 CPM/µl but try a range of probe concentrations the first time) mixed with 19µl of HB8, heat 3min at 95°C, chill on ice; prehybridization (step 6) is at room temperature; hybridization is at 42°C; and steps 9 - 11 are replaced by stringency washes (the RNase treatment is eliminated) appropriate to the melting temperature of the oligomer used (approximately 15°C below the Tm of the probe) (we have used 42°C at 0.1xSSC which can serve as a starting point).
Controls:
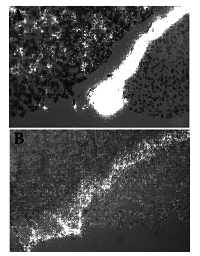
The choice of an appropriate control is the most challenging aspect to using in situ hybridization properly. There are a number of controls that have been used by investigators to establish the validity of their results (Table IV), some are better than others. The best control is common sense. Does the signal make sense to you? Is it localized over the cytoplasm of the cell rather than over the nucleus? Are there negative cells in the tissue as well as positive cells? Is the gene expressed in appropriate sites in control tissues? These are important questions that must be addressed each time the results of an in situ experiment are evaluated. It is easy to assume that any accumulation of silver grains represents a positive signal but careful examination and rehybridization may yield very different results (Figure 1).

Figure 1. Examples of a good (A) and bad (B) in situ hybridization results. Sections of rat pituitary were hybridized with 35S-labeled pro-opiomelanocortin (POMC) riboprobes using two different methods. Panel (A) shows a section hybridized according to the protocol outlined in this review and reveals strong hybridization to the intermediate lobe (central band of positive cells), scattered hybridization to the anterior lobe corticotrophs (individual cells to the left of the intermediate lobe). Panel (B) is the same pituitary hybridized in parallel with the section in panel (A) using a riboprobe procedure outlined in a recent series of publications (Nikol et al 1992; Simons et al 1993; Leclerc et al 1992). Note the high background in the posterior lobe in panel (B) and the lack of any significant hybridization signal in the anterior lobe. In the absence of any additional information, the background in panel (B) may have been assumed to represent positive hybridization and illustrates the problem associated with accepting all hybridization results at face value. Exposure 1 week; Original magnification 50x; Photographed with polarized light epilumenesence (Leitz).
Table IV. Controls for in situ hybridization. Sense/Antisense Co-localization with protein Multiple non-overlapping probes Northern blots Multiple probes RNase/DNase Common Sense!
Sense and antisense hybridizations are a widely used as controls for in situ hybridizations with riboprobes (Figure 2). Riboprobes are synthesized by transcription utilizing the cDNA as a template for probe synthesis. Messenger RNA is normally synthesized from chromosomal DNA in the 3' to 5' direction producing 'sense' mRNA. To make an 'antisense' riboprobe the cDNA insert is subcloned in a transcription vector in the 3' to 5' direction relative to an RNA polymerase initiation site. Transcription takes place in the presence of a labeled nucleotide so that the resulting 'antisense' probe is complementary to the mRNA and will bind to it during the hybridization reaction. Insertion of the cDNA insert in the opposite orientation relative to the RNA polymerase initiation site will transcribe the control 'sense' riboprobe, a sequence identical to the mRNA in the tissues which will not hybridize. Since the 'sense' and 'antisense' probes both contain the same relative proportion of all of the nucleotides (A/U's or G/C's) in the same sequence this has been considered an excellent control for riboprobe hybridizations (Cox et al 1984a).

Figure 2. Transcription of 'sense' and 'antisense' riboprobes for use as controls for in situ hybridization. Cellular mRNA is transcribed by RNA polymerase in the 3' to 5' direction using one strand of the chromosomal DNA as a template. Riboprobe vectors are available (pGEM, Bluescript) which have built in RNA polymerase initiation sites adjacent to a series of unique restriction site used to clone in a desired cDNA. Depending on the orientation of the cDNA insert in the riboprobe vector relative to the SP6 or T7 RNA polymerase initiation sites either 'sense' or 'antisense' RNA probes can be synthesized. Transcription from the cDNA template using SP6 RNA polymerase in this example produces a nucleotide sequence identical to the mRNA otherwise known as the 'sense' transcript. Since the 'sense' probe is the same sequence as the cellular mRNA it will not bind during the hybridization reaction but can be used as a negative control. Transcription of the cDNA template from the opposite end using the T7 RNA polymerase initiation site will produce an 'antisense' RNA probe which will hybridize to the mRNA in the tissues. Antisense transcripts will be generated when the 3' end of the cDNA is closest to the site of transcription initiation.
One word of caution is warranted regarding the use of 'sense' and 'antisense' riboprobes. It is often possible to see positive hybridization or even high backgrounds using 'sense' riboprobes yet find a very strong, specific and reproducible signal in the parallel 'antisense' hybridization. How should this be interpreted? Is there antisense mRNA in the tissue? Is the entire hybridization flawed? The first assumption is that the researcher has done something wrong and the experiment is usually repeated until the 'sense' hybridizations are appropriately negative and the 'antisense' hybridizations appropriately positive. The question remains however was the first or second experiment flawed? There is no easy answer to these questions and the assumption is that 'sense' hybridizations should be negative.
A common approach used in my laboratory is hybridization with multiple probes in a single experiment. This can ensure specificity and provides the maximum amount of information per experiment. Typically four antisense probes are hybridized in a given experiment and the results of each hybridization compared for specificity. The probes should show more than one hybridization pattern for the experiment to succeed and should include at least one probe which will hybridize to a known subset of cells in the sections being examined. A good control for human tissue is von Willebrand's Factor (VWF) which is synthesized only by endothelial cells. Other cell types in the tissue will be, or should be, negative and the researcher can evaluate tissue background using this approach. The use of a positive control probe with a known cellular distribution of hybridization in the tissues also provides a control for positive mRNA hybridization and the efficiency of the experiment. The specificity of the hybridization is indicated when one probe, VWF for example, hybridizes to endothelial cells and another probe hybridizes to other cells in serial sections (Wilcox et al 1988). This is a good control and is generally accepted by journal reviewers.
The mistake people commonly make with this approach is the choice of the control probes. Often tissues are hybridized with poly d(T) to detect poly (A) mRNA tails or with housekeeping genes like actin, known to be expressed by all cells. These are poor choices for controls since the result will be positive hybridization to all cells with no negative cells present to determine the amount of background hybridization. The biggest problem with in situ hybridization is background and determining when accumulation of silver grains is real or not. It is for this reason that it is important to have clear positive and negative hybridizations included in an experiment so that it is clear what each will look like.
Hybridization of cDNA or riboprobes to tissue sections obviously depends on the presence of mRNA in the tissues. It is for this reason that another control that has been used is predigestion of the tissue sections with RNase and DNase. Since RNase but not DNase should degrade the signal this is assumed to confirm the necessity of cellular mRNA for the hybridization reaction. One problem with this approach is its application to hybridization with riboprobes and the possible degradation of the probe itself rather than the tissue mRNA.
Additional controls which have been used include many which fall in the common sense category. Multiple non-overlapping probes encoding the mRNA should each give similar hybridization signals whereas an irrelevant nucleotide sequence should not. This is an especially useful control when using synthetic oligomers for hybridization and it is possible to make a number of different probes. Alternatively non-overlapping 5' and 3' ends of the cDNA can be subcloned and used for hybridization. Depending on the level of gene expression in the tissue it should be possible to detect the mRNA by Northern Blots. This would at least confirm the presence of mRNA in the tissues under study. Of course this assumes that there is enough tissue for a Northern or enough cells expressing the gene so that the mRNA can be detected with this technique. Alternatively co-localization of the protein and mRNA using immunohistochemistry and in situ hybridization on serial or same sections can provide another level of assurance that the in situ hybridization signal is real.
The in situ hybridization signal when using exon specific probes should be found primarily over the cytoplasm of the cell (Fremeau et al 1989; Fremeau et al 1986). This is in contrast to recently published reports examining local gene expression in biopsies of human coronary arteries (Leclerc et al 1992; Nikol et al 1992; Simons et al 1993). These authors accepted nuclear grains as indicating a positive hybridization and go so far as to use the number of grains over the nucleus to quantify the amount of mRNA associated with the cell (Nikol et al 1992; Simons et al 1993). The mRNA signal using exon specific probes should be over the cytoplasm. There will be some spread of grains over the nucleus but 90% or more of the signal normally appears as a cluster of silver grains around the nucleus of the cell. Depending on the cellular morphology it is possible to see some nuclear silver grains. For example monocytes or small macrophages with a very thin cytoplasm often show some grains over the nucleus (Nelken et al 1992). This is probably because the mRNA is concentrated in a restricted cytoplasm of which the major part appearing in a tissue cross section overlies the nucleus. On the other hand one of the most common background problems is nuclear silver grains. Anytime the hybridization is pushed by increasing the probe concentration too high or by dropping the stringency of the final wash the first thing that happens is that all of the cells in the section show a nuclear signal. This does not mean that the mRNA is in every cell but rather that it is time to begin to trouble shoot the experiment to determine the source of background.
Non-Radioactive In Situ Hybridization:
There has been a lot of interest over the last few years in the use of non-radioactive probes for in situ hybridization. The advantage of non-radioactive to radioactive in situ hybridization is the lack of long autoradiographic exposure times, the discrete cellular signal and the potential use of multiple probes within an in situ hybridization experiment on a single slide. This procedure however is not without its problems with respect to sensitivity and possibly specificity. We have been working with two protocols based on biotin- or digoxigenin-labeled riboprobes. While these protocols are listed in this syllabus for your information there may be significant problems associated with these methods with respect to probe specificity. The relative merits of each will be discussed in the presentation.
Size Reduction of Riboprobes:
We and others have pubished the observation that smaller probes (300 bases <) tend to give better in situ hybridization results than longer probes (Wilcox et al 1986; Lawrence, Singer, 1985). However for many years my laboratory had very good results using full length riboprobes for hybridization which ranged in size from 300 to 1200 bases long without any size reduction. Our concern has always been specificity of the hybridization probe. We felt that any attempts to reduce the size of the probes may reduce the specificity of the hybridzation since the probe would be randomly cleaved producing a smear on sizing gels rather than a single band of a particular size. We therefore advocated the use of coctails of shorter probes produced by limiting the size of the cDNA template if sensitivity was an issue. However we never found the need to do this since our hybridization results with probes as long as 1200 bases were satisfactory. We recently looked at this issue in greater detail and reevaluated the effect of size reduction of riboprobes by alkaline hydrolysis on the subsequent in situ hybridization signal.

Figure 3. Effect of alkaline hydrolysis of 35S-POMC riboprobe on probe size and hybridization to rat pituitary sections. A 600 base POMC riboprobe was subjected to alkaline hydrolysis for different periods of time and used for a hybridization probe to frozen sections of rat pituitary. X-ray film autoradiography was used to visualize the size of the probes after gel electrophoresis and the results of the in situ hybridizations. Note the smaller probe preparations showed a lot of size heterogeneity but displayed strong hybridization to the anterior and intermediate lobes of the pituitary without a corresponding increase in background in the posterior lobe.
The method used for alkaline hydrolysis was as previously reported by Angerer and colleagues (Cox et al 1984b; Angerer et al 1987). After transcription the probe is dissolved in 50µl of DEPC-H2O and an equal volume of 0.2M carbonate buffer pH 10.2 (80mM NaHCO3, 120mM Na2CO3 - made fresh or stored in small frozen aliquots) added. The probe is then incubated for varying periods of time at 60oC and the following formula used to determine the approximate probes size (this is by no means exact):
t = (Lo - Lf)/k(Lo)(Lf)
t = time in minutes
k = 0.11 strand scissions/kb/min
Lo = initial length in kb
Lf = desired length in kb
After digestion the probe is neutralized by adding 3µl of 3M NaAc, pH 6.0 and 5µl of 10% (v/v) glacial acetic acid. The probe can then be precipitated and stored under ethanol. X-ray film autoradiography suggests that the hybridization signal is improved using hydrolyzed probes without a corresponding increase in background (Figure 3). We are currently evaluating the results of these hybridizations at the cellular level by emulsion autoradiography. Further work is being done to evaluate the results of hybridization to lower copy number mRNAs.
In Situ PCR:
The most recent modification to the basic in situ hybridization protocol has been the development of in situ PCR. The technique is essentially a PCR reaction performed directly on a tissue section using a modified heating plate which holds microscope slides during the amplification process. The required PCR primers and Taq polymerase are added directly to the tissue sections and the product amplified using standard PCR protocols. This procedure has been described most extensively as a method for the detection of viral DNA particles in tissue sections (Komminoth, Long, 1993; Nuovo et al 1991b; Nuovo et al 1991a). Only recently have a few papers appeared which suggest that this technique may be applied to the amplification of cellular mRNA directly in tissue sections (Patel et al 1994; Heniford et al 1993) or cultured cells (Chen, Fuggle, 1993). There are a number of concerns about the specificity and reproducibility of this technique applied to the detection of mRNA in tissue sections which will be discussed during the presentation. Some indication of the general application of this technique can be deduced from the fact that there are only very few publications using this method beyond the first demonstration of the method.
In Situ PCR or PCR and in situ hybridization?
Given many of the problems associated with in situ PCR for the detection of cellular expression of mRNA directly in tissue sections I have been working towards developing the ability to do quantitative PCR on tissue sections previously prepared for in situ hybridization. I have a large collection of tissues which have been frozen and prepared for our standard in situ hybridization procedure. These tissues have been used over a number of years in studies localizing specific mRNAs by in situ hybridization. We have avoided studies requiring the quantitation of specific mRNAs by in situ hybridization due to the inherent drawbacks in this technique which make quantitative in situ hybridization questionable. However these section and frozen tissue libraries are compatible for solution PCR amplification. PCR can be performed on this material either by scraping tissue off of existing microscope slides or by cutting new frozen tissue sections and collecting these in tubes for homogenization and mRNA extraction. A scaled down RT-PCR amplification is then performed on the mRNA which is used for first strand synthesis and amplification. The combination of in situ hybridization and PCR analysis on the same tissue blocks is potentially an extremely powerful tool for the analysis of local gene expression in histological material. This should allow not only the cellular localization of a specific mRNA but the simultaneous confirmation of that mRNA's presence in the tissue and estimates of mRNA levels.
Home / Part I / Part III / Part IV