2020 Vision
In 2007, Uganda announced a bold plan to eliminate river blindness by 2020. The Carter Center's Moses Katabarwa 97MPH has been in the battle from the beginning—and he believes they're going to win
The River Nile is the longest in the world, moving mightily over more than four thousand miles and through ten African countries before emptying itself into the Mediterranean Sea. For millions it is the source of life and legend, death and mystery, symbol and song—not to mention water, food, transportation, and money. It is at once mythic and utterly real, visible from space and from bridges, banks, and boats.
With all its power, you’d think the source of the Nile would be well established, but pinpointing the beginnings of a force so vast is no simple thing. Although the longer of its two major tributaries, the White Nile, is thought to originate at Lake Victoria—probably in Burundi or Rwanda—many say the Nile has its source in Uganda.
Of course, the river itself pays no mind to what people think about it—like the Mississippi of the song Old Man River, it just keeps rolling along. But Ugandans are proud to claim its possible origins in the country that Winston Churchill famously called the “pearl of Africa” for its lush, landlocked beauty.
As the matriarch of Uganda’s many rivers and streams, the Nile holds innumerable secrets, including a tiny black fly that breeds only in swift-moving waters and carries inside it the makings of a particular sort of human misery: onchocerciasis, or river blindness.
It’s this fly that Moses Katabarwa 97MPH, a Uganda native and senior epidemiologist for The Carter Center’s River Blindness Program, has been chasing for more than twenty years—most recently this summer, when he visited his homeland to conduct field research and attend a national meeting on river blindness.
The black fly Simulium—about the size of a Georgia gnat—is unusual in its preference for moving water, since so many of its brethren pests like to breed in warm, stagnant puddles and ponds. Two different types of the fly carry the river blindness parasite, Onchocerca volvulus—one, S. damnosum, dives into flowing waters to lay its eggs, shooting them from its tiny body bundled in a superglue-like substance that sticks them firmly to underwater rocks or vegetation. The other, S. neavei, can lay eggs only in small river crabs and has a shorter flight range than its wily cousin.
When people are bitten by female flies (the males don’t bite), they can become infected with onchocerciasis microfilaria, pre-larval-stage parasitic worms that wriggle their way around under the skin. Like the Guinea worm parasite—another of The Carter Center’s targeted diseases—these worms can breed inside the body; they multiply and sometimes form writhing nodules that can be felt and even seen.
And they love to migrate up to the eye, where they cause irritation and nerve damage, and eventually, as they die, leave debris that can build up to the point of diminished vision and permanent blindness. Affecting some eighteen million people in Africa and the Americas, the disease is the second-leading cause of preventable blindness in the world.
River blindness infection triggers an immune response similar to that of an allergic reaction, which is why it causes intense itching, swelling, rashes, lesions, and skin discoloration—a pattern commonly referred to as “leopard skin.” Ironically, a strong immune system can produce a more severe reaction.
“If you have an efficient immune system, you will suffer much more,” says Katabarwa. “The more you scratch, the more you want to.”
It takes many fly bites to produce a bad infection—what health workers offhandedly call a high “worm load”—but in rural villages that are situated near swift-moving rivers and streams, it’s not hard to become bait.
The village of Lapaya, in northeast Uganda’s Pader district, is within a half-mile of Aru Falls, a rushing tributary that eventually feeds into the Nile. It’s the home of Ojok Charles, a sixteen-year-old boy with a soft voice and a gentle manner who spends most of his days sweeping the dirt yards and public areas of the village.
When he was twelve, Ojok was out cutting timber to make charcoal that he could then sell to earn money for his school uniform. As he worked for days in the dense brush, he was bitten thousands of times by black flies, contracting a high level of onchocerciasis infection. Ojok describes being able to feel the worms moving in his eyes as the disease progressed. By 2009, Ojok’s vision was so poor that he had to leave school. In 2010 he went completely blind.
Ojok’s mother, Acan Bicentina, has three other children who go to work with her in the fields nearly two miles away. She has to leave Ojok behind each day, and although she tries to provide food for him, she is not always able.
“I have no hope for him,” she says. “He will always depend on me.”
“This is a terrible hardship for a family like this,” says Katabarwa. “You see him, but he is not alone. There are many like him.” An estimated 1.4 million people in Uganda are affected by river blindness, and two million are at risk.
River blindness is endemic to thirty-seven countries in Africa and Latin America, with some 99 percent of cases in Africa. But Uganda is unique: in 2007, the government and its Ministry of Health announced their intention to eliminate the disease completely—something many believed couldn’t be done.
Carter Center leaders hope that bold step will pave the way for other African countries to follow suit. “Moving from control to elimination is a crucial turning point in the fight against river blindness,” says Frank O. Richards, director of the Center’s River Blindness Program. “Once elimination becomes the goal, it is no longer business as usual. A program and its partners must ratchet up interventions.”
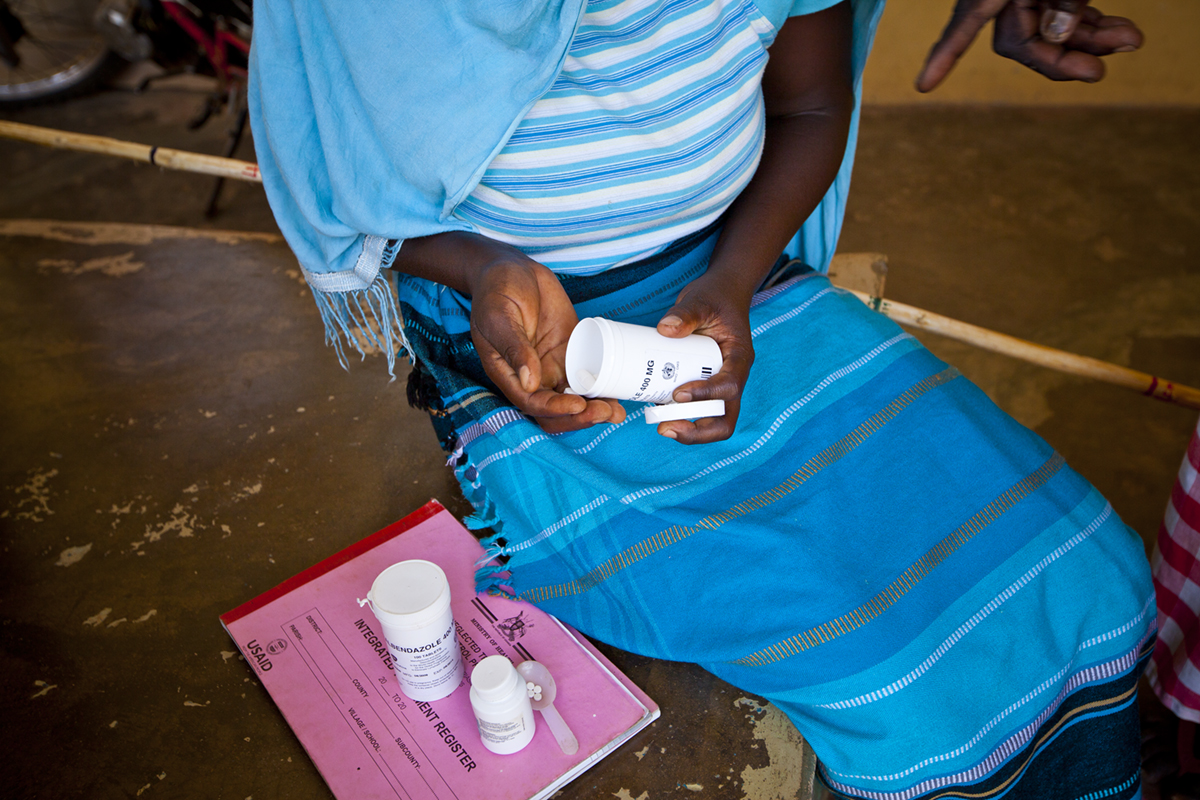
Working in close partnership with The Carter Center, Ugandan health officials are taking a multifaceted approach that includes annual or semiannual treatment with the drug ivermectin, or Mectizan; and vector control and elimination with Abate, a pesticide compound that doesn’t harm humans or the environment.
Pader, one of thirty-five endemic districts, is a relatively new one for the program, which began treatment here in 2009. William Oyet, the Ministry of Health vector control officer for the Pader district, says the rate of blindness is very high. Each year he schedules a day for Mectizan distribution and sends word through village networks. “The people know us now,” he says. “They go very deep into the bush to tell others, ‘This is the one giving out the drug.’ ”
Oyet shows Carter Center visitors the detailed journal in which he records drug recipients. The first year, nearly all 186 residents in his area showed up at the central clearing to receive the medicine; the following year, though, turnout was lower due to the side effects. Because Mectizan kills the onchocerciasis microfilaria in the body, the autoimmune response is temporarily ramped up, causing increased itching and swelling. Last year, community compliance rose again as those affected began to understand the benefits.
Ivermectin is provided by Merck through the Mectizan Donation Program, part of the Emory-affiliated Task Force for Global Health, to treat river blindness and lymphatic filariasis. The program, which provides 140 million doses annually, celebrates its twenty-fifth anniversary this year.
“As the pioneer drug donation program for neglected tropical diseases, our success has had an enormous impact on global health by creating innovative health interventions and by spawning remarkable public-private partnerships,” says Joni Lawrence, associate director of the program.
In remote areas of Uganda where river blindness is most common, treatment with Mectizan is administered at the community level, led by district coordinators like Oyet who organize distribution through kinship structures—the complicated, far-reaching network of family and marital relationships that connects people from village to village.
This public health strategy was pioneered by Katabarwa, who knows from personal experience that in rural communities, one of the most powerful drivers of behavior is the shared obligation to care for those in need.
Traveling in Uganda with Katabarwa is like having a complete guidebook, a botanical field reference, and a dramatic novel set in the country, all wrapped in one. Driving north from the capital city of Kampala, Katabarwa points out the various crops grown in the acres of rich farmland stretching in every direction: corn, millet, cassava. “You can literally grow anything here,” Katabarwa says.
Without preamble, he also points out the area where his older brother’s body was dumped after he was killed by soldiers during the second reign of President Milton Obote, who both preceded and followed the rule of dictator Idi Amin. Katabarwa describes how he later went to the area and enlisted the help of the surrounding community to search for the body. It took two weeks to locate his brother’s bones, which were then given a national burial.
Katabarwa was one of eleven children, raised in southwestern Uganda’s Bushenyi district by parents who were relatively prosperous in dairy and crop farming. His father was a local leader and a devout Anglican Christian, leading the family in prayer and song each evening—often after heated debate over politics and religion.
Unlike most boys of the time, Katabarwa wanted to go to medical school. “I never wanted to be a policeman,” he says, with typical mischievous humor, adding that it was common for boys to equate uniforms with power and seek out such jobs.
As Katabarwa grew up, the widespread tensions and violence created by the Amin and Obote governments rose steadily, eventually pushing his older brothers to form a liberation group that trained in neighboring Tanzania.
That turned out to be bad luck for Katabarwa. When he was in high school, in 1977, he was mistakenly identified by the Ugandan government as one of his brothers involved in revolutionary activity. The government, he says, arranged for him and two schoolmates to be poisoned—enlisting a “friend” to put a deadly neurotoxin in their tea.
Katabarwa survived; his schoolmates were not so fortunate. But he was bedridden for two years and spent a third relearning to walk and reconstructing memories of his life before. By the time he was able to go back and finish high school, his dream of medical school had faded in the light of a new reality.
That’s how Katabarwa wound up studying agronomy at Kampala’s Makarere University, where he completed a research project on the diseases of tea plants. It’s one of the many notable ironies of Katabarwa’s life that he loves tea—which is grown in his home district—with something approaching reverence, despite the fact that it nearly killed him.
After college, Katabarwa took a position with the British nonprofit Oxfam, working with the Karamajong people of northeast Uganda. His charge was to convince the nomadic tribe to resettle in a more hospitable area, plant viable crops, create buildings and roads, and practice basic public health.
Living in a grass hut deep in the bush, Katabarwa found life among the Karamajong wild and exhilarating. “I was young and adventurous, and I didn’t care about the conditions,” he says. “I was enjoying everything.”
But this was also when, as he puts it, the former would-be doctor “accidentally got involved in public health without knowing it.” As he saw the Karamajong suffering from high levels of malaria, tuberculosis, and a host of other diseases—with virtually no access to traditional health care—Katabarwa began to understand the need to educate and empower them to take care of themselves.
He brought in health workers to help teach them the basics of public health, such as hygiene, midwifery, and vaccination. He also began to see the potential for using the kinship model to facilitate community-driven care.
“We were training people with no education to do immunizations,” he says. “But there were no hospitals for miles. They had to be able to do it themselves.”
When Katabarwa met his wife, Lois, he was obliged to leave the jungles of the Karamajong. In 1992, he landed a position with the River Blindness Foundation, a young organization that was expanding efforts to control onchocerciasis through the use of Mectizan. The foundation was cofounded and funded by philanthropist John Moores, who later became board chair for The Carter Center.
Katabarwa rapidly emerged as an early leader in the fight against the disease, helping to establish the foundation’s office and traveling the country to map endemic areas. In those days, he says, many of the affected communities couldn’t be reached by car; he spent days in the field on foot, hacking through the jungle with an axe and shovel to locate areas where river blindness was common.
“We joke that where the road ends is where you find these diseases,” he says.
In 1996, The Carter Center—an Emory institutional partner since it was founded by former US President Jimmy Carter and his wife, Rosalynn, thirty years ago—assumed the activities of the River Blindness Foundation, and leaders helped send Katabarwa to Emory’s Rollins School of Public Health for an MPH. He later earned a PhD in anthropology in the UK. A recipient of Emory’s Sheth Distinguished International Alumni Award, he also serves as adjunct faculty at Rollins.
From 1998 to 2003, he served as country director for The Carter Center’s Uganda office. “When we started, it was just the main building, and one small Suzuki sitting up on blocks,” Katabarwa says, surveying the office, which is just a few blocks from the Ministry of Health.
Katabarwa’s pride is well-founded: the office has expanded to several buildings, a small fleet of Land Cruisers, a scientific research laboratory, and six full-time Carter Center staff, several of whom were recruited by Katabarwa.
Now led by country representative Peace Habomugisha, who has been with the program since 1999, the office is a critical strategic partner for the Ministry of Health and its Onchocerciasis Elimination Expert Advisory Committee—working alongside the Lions Clubs, Sightsavers, the African Program for Onchocerciasis Control, and the World Health Organization, among others.
Habomugisha, a quiet woman who exudes unflappable calm, has become a well-recognized figure among ministry officials and public health leaders. To navigate the complexities of the Ugandan health care system, she must act as manager, facilitator, strategist, and politician—which may be why she prefers field work to the office. “In the communities, you can truly see that you are helping people,” she says.
In July, Katabarwa and Habomugisha met with Dennis Lwamafa, the Ministry of Health’s Commissioner for Health Services, National Disease Control. The three discussed the positive progress of the elimination effort. But they also agreed on the need to continue surveillance and even intensify efforts in remote areas.
“Today, we have interrupted transmission in several areas, but challenges remain,” Lwamafa said. “We must be prepared for the long haul as we see the fruits of our efforts over time. We really value this friendship with The Carter Center.”
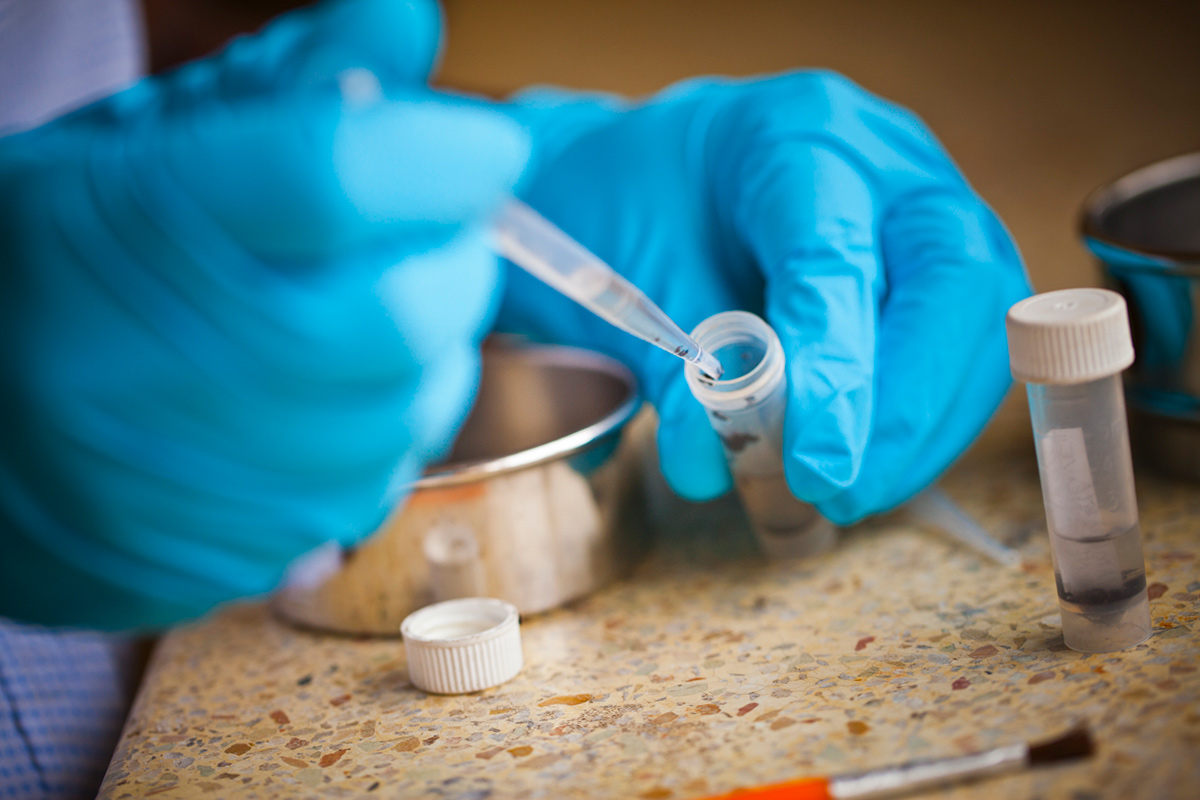
A critical part of that ongoing surveillance takes place in the lab located on the second floor of The Carter Center offices. To the untrained eye, it doesn’t look terribly high-tech—but this lab is specially equipped to perform two meticulous processes. One is the analysis of blood samples using the OV-16 antigen to detect exposure to onchocerciasis microfilaria; the other is the testing of black flies and “skin snips,” or human tissue, to learn whether they contain the DNA of the parasite.
Katabarwa estimates that this lab has performed more OV-16 analysis than any other in the world. Overseeing this enterprise is David Oguttu, senior lab technician, who studied biomedical lab technology at Makerere University. Oguttu was sponsored by The Carter Center in 2007 to travel to the US for a five-week program in practical molecular epidemiology, training with noted expert Thomas Unnasch of the University of South Florida.
Oguttu learned lab techniques including OV-16 testing and DNA analysis using a special machine that creates a polymerase chain reaction. When he returned to Kampala, he trained eight other technicians to conduct these procedures, including laboratory staff Monica Ngabirano and Christine Nahabwe.
Both these methods of analysis are extremely sensitive, which is a distinct advantage as rates of onchocerciasis exposure become low. Oguttu and the lab staff are seeing fewer positive responses as transmission is reduced in some areas of the country. But, he says, the statistical data they are producing in the lab is more important than ever as the country strives for elimination by 2020.
“We are the only lab doing this in Uganda,” Oguttu says. “These are neglected tropical diseases. No one cares about them. We are the only department going out into the communities to bring these services to the people. This is great work, and I am very grateful to be doing it.”
In the district of Hoima, one of the areas longest-targeted for river blindness control and now elimination, memories of the disease are already fading into the past.
As guests from The Carter Center approach Nyabuhuku Village for a site visit, there is a ripple of excitement among the villagers gathered in the central area under the trees. The visitors are welcomed by the local council chairman and formal greetings are exchanged. When leaders ask the assembled crowd about the impact of continuous treatment, hands fly up in response: the villagers eagerly describe the itchiness, swelling, poor vision, and rough, discolored skin that once affected most of them.
But since they have been receiving Mectizan twice a year, those symptoms are mostly gone. Owinji Charles, who is fifty-seven and a farmer, says he had trouble working for years due to river blindness, but then began receiving treatment; when he relocated to this village, where distribution occurs twice a year rather than once, he got better even faster.
“He says it is a positive experience,” translates Ochumu Morris, district coordinator for the nearby Buliisa district. “The itching stopped and he was not inconvenienced anymore. He is now comfortable, a free man.”
In a complementary effort, explains Morris, nearby water sources have been dosed with Abate—a chemical donated by BASF—to kill the black flies that carry the disease. These flies are the type that require small river crabs for breeding and can’t fly very far, so elimination efforts are highly productive. As part of ongoing quarterly monitoring, hundreds of crabs are regularly trapped and checked for the presence of fly larvae or pupae. Treatment of the water began in 2009, and by late 2010, it was stopped because no more flies were found.
“We stopped because it was so successful,” says Morris, a vector control officer for the Ministry of Health. “We have to continue monitoring for two years, but we are optimistic because the chemical was very effective. It looks promising.”
Katabarwa tells the villagers of Nyabuhuku that the intervention efforts have worked. “Your children will never know this terrible disease,” he says.
This area will soon join the country’s three others where transmission of river blindness has officially been declared interrupted—a major step toward the goal of nationwide elimination.
“We have started with the easy places, and are moving on to harder areas,” says Stella Agunyo, a data analyst who has worked in The Carter Center’s Uganda field office since 2003. “It is more difficult in places such as Pader, where we do not have enough information from fly-catching sites.”
Agunyo goes out into the field about four times a year to conduct research and surveillance. Fluent in several local languages, she says she relishes the opportunity to share information and hear stories face-to-face—visiting communities, interacting with local people, and doing the ground-level research that is essential to the River Blindness Program’s success.
“We need to work closely with the districts to make sure they are following the protocol,” she says.
For that, they depend on the broad network of local health workers. Nicholas Ogweng, district coordinator for Moyo and nearby Adjumani, has worked as a vector control officer for the Ministry of Health for more than fourteen years. During that time, the incidence of river blindness in his area has gone from 90 percent of the population to less than one percent.
“This has been a very positive intervention for these communities,” he says. “There is almost no leopard skin or blindness now.”
Still, scattered cases remain—as do scars.
In Moyo’s Liwa Village North, James Waya sits in the shade of a tree so broad its lowest branches nearly touch the ground, tending a fire where he does metalwork by using the red-hot embers to shape spears and other tools. At sixty-seven, Waya has lived a lifetime of hardship. His lips and ears were chopped off by rebels during the rule of Amin, whose shadow still looms over the country.
And his legs are permanently marked by the extreme leopard skin pattern that is a sign of severe, prolonged infection with river blindness. Waya describes suffering for years from the itching and agony that accompany the disease, which he believed he had contracted from stepping in elephant dung.
After seven years of treatment with Mectizan, his symptoms have receded.
“Now,” he says, “my suffering is simply getting old.”